What high-value potential applications does titanium carbide have after nanometerization?
The well-known titanium carbide (TiC) is a typical transition metal carbide, with high melting point, high hardness, high Young's modulus, high chemical stability, wear and corrosion resistance, good electrical and thermal conductivity and other characteristics, so It has a wide range of uses and great potential in cutting tools, aerospace components, wear-resistant coatings, foam ceramics and infrared radiation ceramic materials.
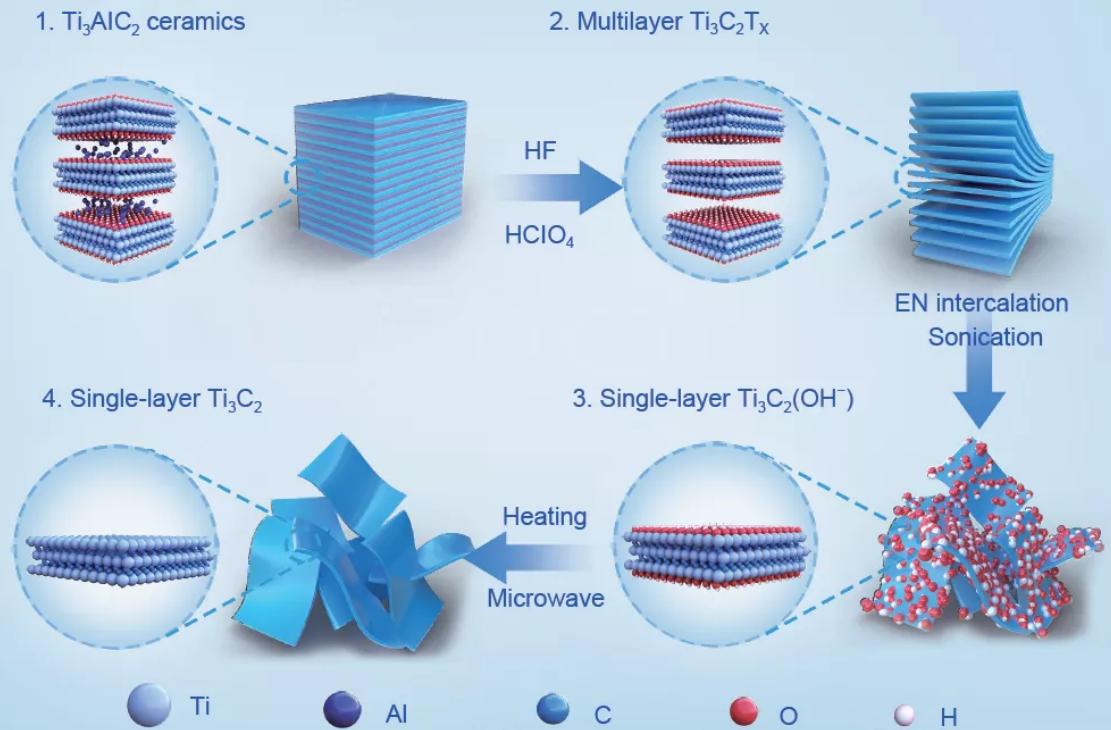
However, with the development of research in recent years, two-dimensional titanium carbide nanomaterials have gradually attracted attention, which is a graphene-like structure with high application value. This structure is composed of transition metal titanium carbide with a thickness of several atomic layers. The chemical formula is Tin+1CnTx (n=1~3). T stands for chemical functional groups (such as -OH, -O and -F). Common titanium carbide materials There are Ti3C2, Ti2C, TiC, etc.
Since the research group of Professor Gogotsi of Drexel University in the United States reported on the first Ti3C2 material in 2011, titanium carbide material has been used in lithium/sodium due to its excellent electrical conductivity, atomic layer thickness, hydrophilicity, and high electronegativity. Ion batteries, capacitors, catalysis and sensing have been widely used. At present, the research on titanium carbide mainly focuses on the preparation and application of ultra-thin nanosheets.
Nano titanium carbide material
However, research has found that titanium carbide nanosheets have a drawback, that is, they are prone to stacking during use, which is not conducive to ion/electron transmission.
In contrast, one-dimensional nanomaterials derived from titanium carbide can effectively overcome the above shortcomings, and exhibit the advantages of large aspect ratio, high specific surface area, and more active sites, thereby effectively avoiding the close packing of active materials. , Improve the ion/electron directional conductivity, shorten the response time and extend the cycle life.
Therefore, one-dimensional nanomaterials derived from titanium carbide starting materials have become the main research direction, including titanium carbide (Ti3C2) nanobelts, titanate nanobelts and titanium dioxide (TiO2) nanowires.
Titanium carbide-derived one-dimensional nanomaterials
In the current research, the derivatization and transformation process using two-dimensional Ti3C2 as the starting material to transform into one-dimensional nanostructures mainly involves two types of mechanisms.
The first type of mechanism: Under the action of oscillation or hydrolysis of the two-dimensional titanium carbide, first defect sites are generated on the edge of the nanosheet, and then under the action of shearing force, the Ti-C bond is broken, and then a one-dimensional nanostructure is formed;
The second type of mechanism: Ti atoms on the surface of titanium carbide are first oxidized to generate TiO2 nanoparticles, and then alkalized to generate titanate nanowires or epitaxially grow to prepare TiO2 nanowires.
1. Titanium carbide (Ti3C2) nanobelt
Two-dimensional layered titanium carbide (Ti3C2) has the advantages of good electrical conductivity, high structural stability, and rich surface chemical properties. It is used in the fields of energy storage, electrocatalysis, photocatalysis, photoelectric detection, sensing, and regenerative medicine. Broad application prospects. One-dimensional Ti3C2 nanostructures can exhibit many performance advantages over two-dimensional ultra-thin nanosheets. When one-dimensional Ti3C2 nanoribbons are agglomerated, a three-dimensional porous network framework can be generated, providing larger ion transmission channels, and promoting electrons/ions. transmission.
2. Titanate Nanobelt
Alkali metal titanate can be used as electronic materials, optical materials, biomedical materials, etc. because of its good biocompatibility, thermal stability, high mechanical strength, etc. Among them, one-dimensional alkali metal titanate nanomaterials It has unique advantages such as electron transport behavior, optical properties, electrochemical properties, mechanical and mechanical properties, and exhibits enhanced performance in the fields of catalysis, hydrogen storage, chemical sensors, ion exchange, and molecular adsorption.
Using an accordion-like Ti3C2 block as a raw material, it was prepared by derivatizing one-dimensional ultra-thin sodium titanate (NaTi1.5O8.3, M-NTO) and potassium titanate (K2Ti4O9, M-KTO) nanobelts.
3. Titanium dioxide (TiO2) nanowires
TiO2 is a kind of multifunctional n-type semiconductor material. It has the advantages of excellent hydrophilicity, good chemical stability, low cost, non-toxicity, and environmental friendliness. It is widely used in photocatalysis, photoelectric conversion, sensors, energy storage, etc. field. One-dimensional TiO2 nanowires combine the many advantages of one-dimensional materials and TiO2, showing excellent performance.
Room temperature solution phase oxidation is the main method for preparing Ti3C2-derived one-dimensional TiO2 nanowires. Ti atoms on the surface of titanium carbide are first oxidized to form TiO2 nanoparticles, and then epitaxially grown to prepare TiO2 nanowires.
Application of one-dimensional nanomaterials derived from titanium carbide nanowires
One-dimensional nanomaterials derived from Ti3C2 are currently mainly concentrated in the three fields of energy storage, electrocatalysis and sensing. Therefore, the application of these materials still has certain limitations. In future research, the application of one-dimensional nanomaterials derived from Ti3C2 can be extended to other fields, and this one-dimensional transformation can also be extended to the derivation of carbide materials such as Sc2C, Hf2C, W2C, Ti2C, and its preparation and application It has the advantages of simple process, low energy consumption, high preparation efficiency, and diverse product properties. With in-depth research on the direction, it is expected to realize the preparation of one-dimensional nanomaterials of any composition, and promote and deepen its application in many fields.
However, with the development of research in recent years, two-dimensional titanium carbide nanomaterials have gradually attracted attention, which is a graphene-like structure with high application value. This structure is composed of transition metal titanium carbide with a thickness of several atomic layers. The chemical formula is Tin+1CnTx (n=1~3). T stands for chemical functional groups (such as -OH, -O and -F). Common titanium carbide materials There are Ti3C2, Ti2C, TiC, etc.
Since the research group of Professor Gogotsi of Drexel University in the United States reported on the first Ti3C2 material in 2011, titanium carbide material has been used in lithium/sodium due to its excellent electrical conductivity, atomic layer thickness, hydrophilicity, and high electronegativity. Ion batteries, capacitors, catalysis and sensing have been widely used. At present, the research on titanium carbide mainly focuses on the preparation and application of ultra-thin nanosheets.
Nano titanium carbide material
However, research has found that titanium carbide nanosheets have a drawback, that is, they are prone to stacking during use, which is not conducive to ion/electron transmission.
In contrast, one-dimensional nanomaterials derived from titanium carbide can effectively overcome the above shortcomings, and exhibit the advantages of large aspect ratio, high specific surface area, and more active sites, thereby effectively avoiding the close packing of active materials. , Improve the ion/electron directional conductivity, shorten the response time and extend the cycle life.
Therefore, one-dimensional nanomaterials derived from titanium carbide starting materials have become the main research direction, including titanium carbide (Ti3C2) nanobelts, titanate nanobelts and titanium dioxide (TiO2) nanowires.
Titanium carbide-derived one-dimensional nanomaterials
In the current research, the derivatization and transformation process using two-dimensional Ti3C2 as the starting material to transform into one-dimensional nanostructures mainly involves two types of mechanisms.
The first type of mechanism: Under the action of oscillation or hydrolysis of the two-dimensional titanium carbide, first defect sites are generated on the edge of the nanosheet, and then under the action of shearing force, the Ti-C bond is broken, and then a one-dimensional nanostructure is formed;
The second type of mechanism: Ti atoms on the surface of titanium carbide are first oxidized to generate TiO2 nanoparticles, and then alkalized to generate titanate nanowires or epitaxially grow to prepare TiO2 nanowires.
1. Titanium carbide (Ti3C2) nanobelt
Two-dimensional layered titanium carbide (Ti3C2) has the advantages of good electrical conductivity, high structural stability, and rich surface chemical properties. It is used in the fields of energy storage, electrocatalysis, photocatalysis, photoelectric detection, sensing, and regenerative medicine. Broad application prospects. One-dimensional Ti3C2 nanostructures can exhibit many performance advantages over two-dimensional ultra-thin nanosheets. When one-dimensional Ti3C2 nanoribbons are agglomerated, a three-dimensional porous network framework can be generated, providing larger ion transmission channels, and promoting electrons/ions. transmission.

2. Titanate Nanobelt
Alkali metal titanate can be used as electronic materials, optical materials, biomedical materials, etc. because of its good biocompatibility, thermal stability, high mechanical strength, etc. Among them, one-dimensional alkali metal titanate nanomaterials It has unique advantages such as electron transport behavior, optical properties, electrochemical properties, mechanical and mechanical properties, and exhibits enhanced performance in the fields of catalysis, hydrogen storage, chemical sensors, ion exchange, and molecular adsorption.
Using an accordion-like Ti3C2 block as a raw material, it was prepared by derivatizing one-dimensional ultra-thin sodium titanate (NaTi1.5O8.3, M-NTO) and potassium titanate (K2Ti4O9, M-KTO) nanobelts.
3. Titanium dioxide (TiO2) nanowires
TiO2 is a kind of multifunctional n-type semiconductor material. It has the advantages of excellent hydrophilicity, good chemical stability, low cost, non-toxicity, and environmental friendliness. It is widely used in photocatalysis, photoelectric conversion, sensors, energy storage, etc. field. One-dimensional TiO2 nanowires combine the many advantages of one-dimensional materials and TiO2, showing excellent performance.
Room temperature solution phase oxidation is the main method for preparing Ti3C2-derived one-dimensional TiO2 nanowires. Ti atoms on the surface of titanium carbide are first oxidized to form TiO2 nanoparticles, and then epitaxially grown to prepare TiO2 nanowires.
Application of one-dimensional nanomaterials derived from titanium carbide nanowires
One-dimensional nanomaterials derived from Ti3C2 are currently mainly concentrated in the three fields of energy storage, electrocatalysis and sensing. Therefore, the application of these materials still has certain limitations. In future research, the application of one-dimensional nanomaterials derived from Ti3C2 can be extended to other fields, and this one-dimensional transformation can also be extended to the derivation of carbide materials such as Sc2C, Hf2C, W2C, Ti2C, and its preparation and application It has the advantages of simple process, low energy consumption, high preparation efficiency, and diverse product properties. With in-depth research on the direction, it is expected to realize the preparation of one-dimensional nanomaterials of any composition, and promote and deepen its application in many fields.















