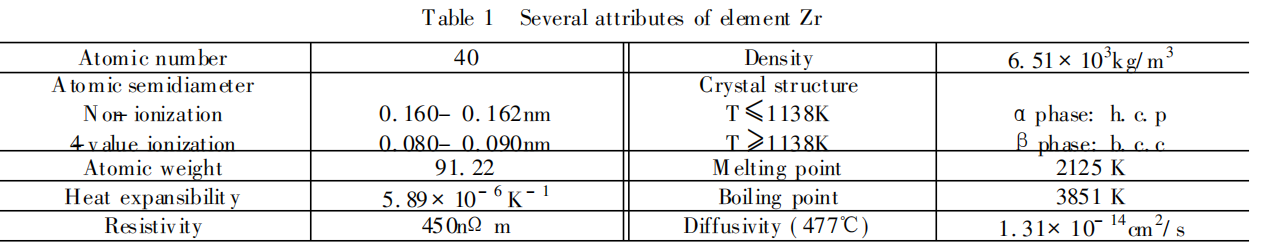
physical properties of metal zirconium
Zirconium is located in the second transition system of group B in the periodic table. The electronic structure is [Kr]4d25S2. Zirconium metal has allotropy, there are low temperature variants (close-packed hexagonal structure) and high temperature variants (body-centered cubic structure), and the transition temperature is 862. Part of the physical properties of metal zirconium are shown in Table 1.
Zirconium metal is an important strategic material with good plasticity and strong corrosion resistance. Zirconium also has special nuclear properties, good radiation resistance, and zirconium still has good gettering properties at high temperatures, making it an important material for atomic energy, electronics, chemical industry, metallurgy, and defense.
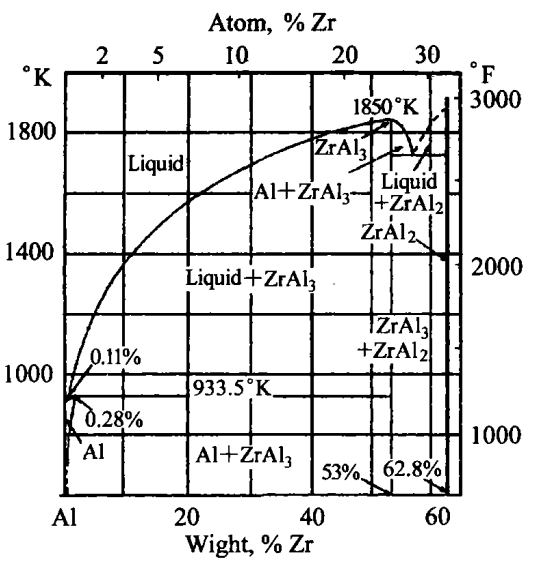
Zirconium can become the crystalline core of aluminum without cooling, but the grain refinement effect of Zr is slightly smaller than that of titanium. The most important role of zirconium is to affect the recrystallization of aluminum, which can increase the recrystallization temperature of aluminum by 100K. When Zr exists in the form of very fine precipitates, its effect is most significant. Rapid cooling during crystallization, followed by precipitation at high temperature, can obtain fine and uniform Al3Zr distribution. The A-phase diagram is shown in Figure 1.

Zirconium metal is an important strategic material with good plasticity and strong corrosion resistance. Zirconium also has special nuclear properties, good radiation resistance, and zirconium still has good gettering properties at high temperatures, making it an important material for atomic energy, electronics, chemical industry, metallurgy, and defense.
Zirconium can become the crystalline core of aluminum without cooling, but the grain refinement effect of Zr is slightly smaller than that of titanium. The most important role of zirconium is to affect the recrystallization of aluminum, which can increase the recrystallization temperature of aluminum by 100K. When Zr exists in the form of very fine precipitates, its effect is most significant. Rapid cooling during crystallization, followed by precipitation at high temperature, can obtain fine and uniform Al3Zr distribution. The A-phase diagram is shown in Figure 1.

related news
-
.png) Nov 11, 2021Aluminum Matrix Composites Reinforced by Titanium Diboride Particles
Nov 11, 2021Aluminum Matrix Composites Reinforced by Titanium Diboride Particles -
 Dec 02, 2021Preparation and application of titanium carbide-based cermet
Dec 02, 2021Preparation and application of titanium carbide-based cermet -
Mar 29, 2022Preparation method of titanium diboride
-
 Dec 13, 2021Effect of Mo–Si–B on Sintering Process and Properties of ZrB2 Ultra-high-Temperature Ceramics
Dec 13, 2021Effect of Mo–Si–B on Sintering Process and Properties of ZrB2 Ultra-high-Temperature Ceramics











